This means that a soap bubble will 'feel' an electric field close to it.

A water molecule (right) like a magnet (left) has polarity. Both have a positive and a negative pole. The difference is that the magnet has magnetic poles, while the water molecule has electric poles.
To understand why water molecules have poles, we should focus on the elements the molecule consists of. Water is made of atoms, and atoms consist of a nucleus and a cloud of electrons surrounding the nucleus. The nucleus is electrically positive while the electrons are negative. The oxygen nucleus is large compared to the two hydrogen nuclei. The large oxygen atom will attract a larger part of the surrounding electron cloud than the smaller hydrogen nuclei. The electron cloud in a water molecule will for this reason be shifted towards the oxygen side of the molecule. This results in a negative pole at the oxygen side, and a positive pole at the hydrogen side. Because the water molecules have polarity, they will behave similar to magnets; they will attract each other just as magnets do.
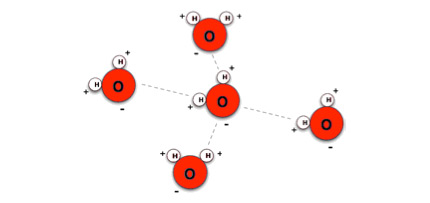
Water molecules attract each other because of their polarity.
Now we are ready to understand the surface tension of water. A water molecule in the middle of a glass of water, will feel the attractive force from all sides by the neighbouring water molecules surrounding it. It will be pulled just as much from above, from below, from the left and from the right side, and all these attractions will cancel out each other. The result is that the molecule is not feeling a net force in any particular direction. Water molecules in the surface, will only feel attraction from three sides; from the right, from the left and from below, but not from above the liquid surface, because there are no water molecules here. The forces from the left and from the right will cancel out each other, but there is no force to equal the force from below. The net force on a surface molecule is therefore down into the liquid. You can say that the water is always trying to pull its molecules away from the surface. The water will try to shape its surface, so that a minimum number of molecules are situated here. In other words: Water will minimize its surface area. This phenomenon is what we call surface tension.
The surface area of a glass of water is minimized when it is flat. If you make a small curvature in the surface, as when a strider is sitting on the surface, the polar forces in the water will try to equal out this curvature again. A strider is not floating in the water, but is carried by the surface tension. It is also surface tension that determines the shape of droplets, water bubbles, soap bubbles, and, it is surface tension that holds a soap bubble together. Surface tension will always work to minimize the surface area of a liquid e.g. soap bubble mixture. The soap film in a soap bubble will feel a force in direction from the centre of the bubble and outward because of the air pressure inside the bubble. The surface tension on the other hand will try to minimize the size of the bubble, to achieve the smallest possible surface area. The soap film will be pushed inwards against the air trapped inside, resulting in a balance between the air pressure and the pressure from the surface tension in the soap film.

A strider is hunting flies on the surface of water. It can run on the surface of water, because surface tension is pushing it upwards.
Sådan kan man måle overfaldespænding
The figure presents a metal tool with two thin rods which can be lowered into a liquid. If the tool is dipped into a liquid and pulled up again a vertical film of the liquid will form on the tool (see the figure). The surface tension of the liquid will make the liquid resist the upward pull when trying to minimize the surface area.

Experimental setup for measuring surface tension.
The work done when pulling the tool out of the surface can be described mathematically as a constant, times the area of the liquid film:
We multiply by 2 since the soap film has two surfaces. The constant is the surface tension, and the equation can now be used to measure its value.
You can use a balance to measure the weight of the tool when it is pulled from the liquid. The liquid will pull the tool downward because of the surface tension, and one should therefore continue to add masses to the balance until the liquid film on the tool breaks. We can now calculate the surface tension of the liquid:
By isolating in the equation above and by inserting the definition of work (), you can calculate the surface tension of the liquid:
Where m is the measured mass and g = 9.8 m/s^2. From the equation it can be seen that the surface tension is measured in the SI-units N/m (Newton divided by meter). Often however, the unit dyne/cm is used. The table presents examples of surface tension measurements from different liquids measured by Soapbubble.dk. It is seen that the surface tension is different for different liquids. The water with soap has a lower surface tension than pure water. Notice that these measurements are not very precise. The table value for the surface tension of water is for example approximately 70 dyne/cm.
Measured surface tension
- Demineralised water: 39.8 den/cm
- Contaminated water (*): 32.1 dyn/cm
- Water with soap: 30.8 dyn/cm
- Alcohol: 27.4 dyn/cm
- *The water was contaminated by sticking fingers into it just before the measurement.