Recipes for soap bubble mixture
The best recipes for mixtures for small and large soap bubbles that last longer

Most good soap bubble recipes mention glycerin. The Glycerin (or glycerol) improves the soap bubble mixture in two ways: It increases the lifetime of the bubbles, and it makes the bubbles more supple.
Water is continuously evaporating from a soap bubble. As a result of this process the soap film becomes thinner and thinner until it breaks. The glycerin in the bubble mixture will stay right at the soap film surface, and will therefore reduce the number of water molecules at the surface. As a result the evaporation will be slower in a soap film with glycerin, because it is always water from the surface that disappears from the soap film during the evaporation process. The glycerin molecules attract water molecules and form weak chemical bonds, the so-called hydrogen bonds. These bonds make it more difficult for the water molecules to leave the surface. One can say that it gets more difficult for the water to evaporate, since the glycerin is pulling the water back into the soap film. For these reasons soap bubbles will generally have a longer life span if they contain glycerin.
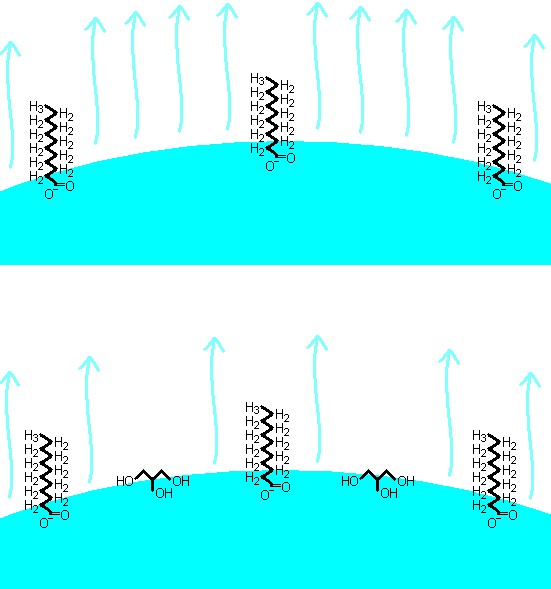
Top: Soap film without glycerin. The film only contains water and soap molecules. The evaporation of water from the film is relatively large. Bottom: Soap film with glycerin. The glycerin is positioned at the surface of the film and thereby inhibits the evaporation of water.
It is said that the glycerin makes a soap film suppler, more flexible. The explanation is supposedly that the glycerin molecules will go in-between the soap molecules. When the film is bending, the long (and charged) soap molecules will move around the smaller glycerin molecules instead of moving around other big (and charged) soap molecules. This gives that soap molecules more freedom of movement and therefore makes the film more flexible.
According to many soap bubble recipes, the bubble mixture should be prepared hours before use. An explanation for this could be that the glycerin needs time to become packed in-between the soap molecules.

The best recipes for mixtures for small and large soap bubbles that last longer

The surface tension in soap bubbles is increased by the water and decreased by the soap
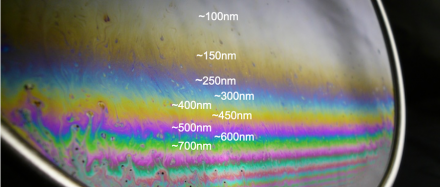
The colors of the soap bubbles come from the phenomenon, thin film interference